Interview with Dr. John R. Hawse from Mayo Clinic
My name is John Hawse. I'm an associate professor at the Mayo Clinic in Rochester, Minnesota and my laboratory is interested in studying estrogen receptors and their functions in breast cancer. As most all of you probably know, the majority of breast cancers express estrogen receptor alpha. Up to 70 to 80% of all breast tumors are ER alpha positive. And for these tumors, we have very good frontline therapies. But my laboratory and others over the past 10 to 15 years have been interested in trying to understand the functions of a second form of the estrogen receptor known as estrogen receptor beta. This is a separate gene that's encoded on a different chromosome from ER alpha, but it is the most closely related protein to ER alpha.
What we've been able to show, through a series of studies over the past years, is that estrogen receptor beta's function in multiple tissue types is different than that of ER alpha, both when it's bound to its naturally occurring ligand, estradiol, as well as when it's bound to a number of synthetic drugs that are used clinically, such as Tamoxifen and Raloxifene. Knowing that estrogen receptor beta can function differently than estrogen receptor alpha, we wanted to start screening breast tumors to determine what percent of them may actually express ER beta.
What we've been able to show through a series of studies here at the Mayo Clinic, in screening over 1,000 breast tumor samples, is that approximately 30% of all breast cancers do express estrogen receptor beta. And this ER beta expression in breast tumors can occur in all subtypes of breast cancers, so the ER beta expression is found in ER alpha positive tumors, it's found in HER2+ tumors. But one of the things that we're most excited about is we've been able to show that approximately 30% of triple negative breast cancers also express estrogen receptor beta.
Triple negative breast cancer is defined by the lack of expression of ER alpha and a couple of other therapeutic targets that are used clinically to treat breast cancer being HER2 and also the progesterone receptor. And for the subset of patients that are diagnosed with triple negative breast cancer, their therapeutic treatment options are very limited. At present, we only treat those individuals with a series of chemotherapy regimens followed by radiation and/or surgery. After that, we really have no follow-up therapies or targeted therapies, so to speak, that can specifically target an individual gene or protein to prevent a recurrence in these individuals.
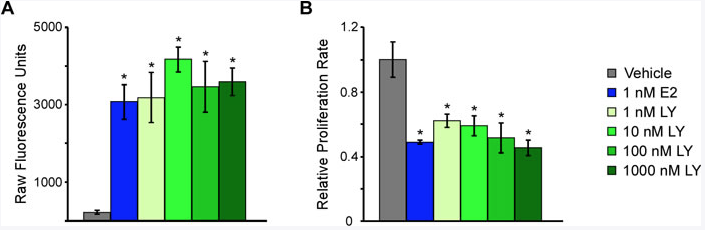
So given that we now know that 30% of triple negative breast tumors express estrogen receptor beta, one of the things that my laboratory is really interested in is trying to define the functions of estrogen receptor beta in this subset of breast cancer patients and to determine if we can actually therapeutically target ER beta like we do ER alpha. And if so, what are the best drugs that we should be using to target this receptor?
So in our recently-published manuscript, what we have been able to do is to develop a number of cell line models systems, using triple negative breast cancer cells in the laboratory, that over-express estrogen receptor beta. And we have shown that treatment of these cells with estrogen or other synthetic molecules that act as agonists for ER beta actually are able to suppress the proliferation of the cells.
And so this was very exciting to us, but counterintuitive a little bit as well, given that estrogen is known to promote the growth of ER alpha positive tumors. But here we're seeing that estrogen actually suppresses the growth of ER beta positive tumors, or at least ER beta positive triple negative breast cancer or cell line model systems.
And so in our manuscript, we tried to begin to understand some of the molecular mechanisms by which estrogen receptor beta is functioning to act as a tumor suppressor in this subtype of the disease. And what we were able to demonstrate is that both in vitro and in vivo, using mouse model systems, that compounds that specifically activate the functions of the ER beta do suppress proliferation, and they also suppress tumor growth. And through a series of studies and understanding some of the gene expression profiles that are differentially regulated, we were able to identify a set of genes that are involved in cell cycle progression.
And then particularly, we showed that cyclin-dependent kinase 1 and cyclin-dependent kinase 7, their expression and function were inhibited by ER beta in triple negative breast cancer cells. And we believe that the inhibition of these two particular genes, as well as the ligands, the cyclin molecules that activate them, may be part of the mechanisms through which ER beta is able to act as a tumor suppressor in triple negative breast cancer.
But as an extension of those studies and going beyond the functions of just estrogen receptor beta, we were also able to show that these particular kinases, CDK1 and CDK7, may independently act as novel therapeutic targets in triple negative breast cancer patients as well. We showed this by specifically knocking down their expression levels using SIR-nase or by using drugs that selectively target these two kinases to inhibit their functions. Under both of these conditions, we were also able to show that suppression of CDK1 and/or CDK7 expression and/or function was able to potently inhibit triple negative breast cancer cell [inaudible].
And so going forward, we plan to continue to follow-up on our studies, trying to continue to further understand the mechanisms of estrogen receptor beta function in triple negative breast cancer patients. We are developing and getting ready to start up a clinical trial that will be aimed at selectively targeting estrogen receptor beta in triple negative breast cancer patients that will be open here at the Mayo Clinic and hopefully other institutions across the country the coming year.
The idea is to screen patients with metastatic triple negative breast cancer for ER beta positive disease. And then those individuals will be eligible to enroll and be treated with the 17 beta estradiol, the naturally-occurring ligand for this receptor that we've been able to show can inhibit the proliferation of triple negative breast cancer cells as well as the progression of triple negative breast cancer in mouse model systems.
Other future studies could involve further exploring the potential to target the CDK1 and CDK7 molecules that we've shown were particularly important in ER beta's mechanism of action in this subtype of breast cancer as well.
Click here to read the full study published by Oncotarget.
—
